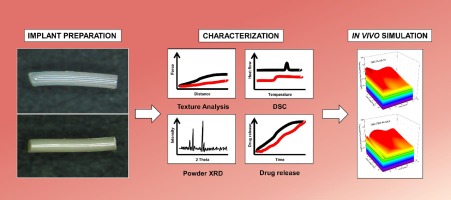
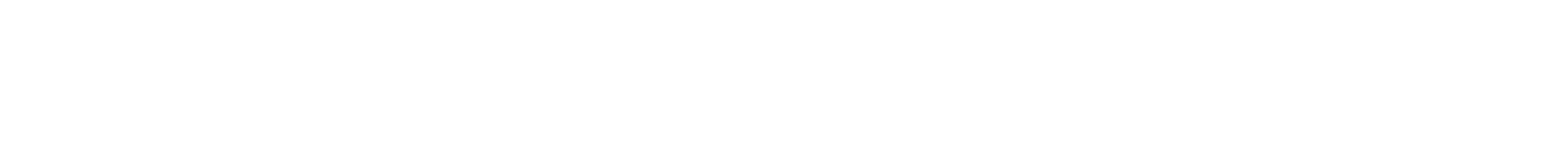Characterization of PLGA versus PEG-PLGA intracochlear drug delivery implants: Degradation kinetics, morphological changes, and pH alterations
Lehner, E. et. al., Journal of Drug Delivery Science and Technology 2024, Volume 99, 105972, https://doi.org/10.1016/j.jddst.2024.105972
Drug delivery to the inner ear presents a unique challenge due to the complex inner ear anatomy and its tight physiological barriers. This study investigates the degradation behavior of intracochlear drug delivery implants (IDDI) composed of dexamethasone and poly(lactic-co-glycolic acid) (PLGA) or polyethylene glycol–poly(lactic-co-glycolic acid) (PEG-PLGA), respectively. IDDI were incubated in artificial perilymph and implants’ degradation kinetics, morphological changes, water uptake behavior, and pH alterations were assessed. Microscopy revealed significant changes in appearance, with PLGA IDDI exhibiting rapid expansion, reaching up to 183 % in diameter and 185 % in length. PEG-PLGA implants showed gradual expansion, reaching a maximum of 178 % in diameter and 144 % in length. Despite these morphological changes, the IDDIs could still be applicable in terms of cochlear dimensions in combination with cochlear implants (CI) in humans or in a domestic pig animal model. © 2024 The Authors. Published by Elsevier B.V.