Bifunctional Peptide-Polymer Conjugates Based Fibers via a One-Pot Tandem Disulfide Reduction Coupled to a Thio-Bromo “Click” Reaction
Kumar, S., et al. ACS Omega, 2020, 5 (30) 19020-19028 https://doi.org/10.1021/acsomega.0c02326
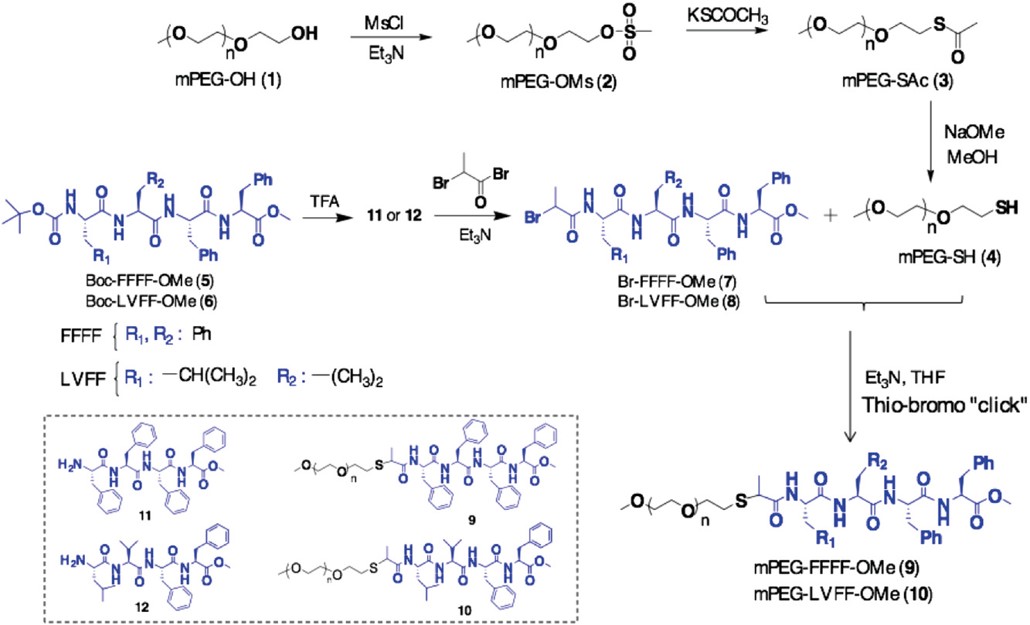
In view of the potential applications of fibers towards material sciences and biomedicine, an effective synthetic strategy is described to construct the bi-headed peptide-conjugate FFFF-PEG-FFFF (Mn,GPC= 3800 g mol-1, Ɖ = 1.10) via a one-pot, tandem–disulfide-reduction coupled to a thio-bromo“click” reaction for supramolecular self-association in solution. The conjugate was investigated via transmission electron microscopy to exploit supramolecular fibril formation and solvent dependent structuring into macroscale fibers via fibril-fibril interactions and inter-fibril cross-linking induced bundling. This synthetic approach opens the way for a simplified synthesis of PEG-containing peptide conjugates. Copyright © 2020 American Chemical Society