Lipid-Polymer Hybrid-Vesicles Interrupt Nucleation of Amyloid Fibrillation
Sen, N. et. al. RSC Chemical Biology, 2024, 5, 1248-1258, https://pubs.rsc.org/en/Content/ArticleLanding/2024/CB/D4CB00217B
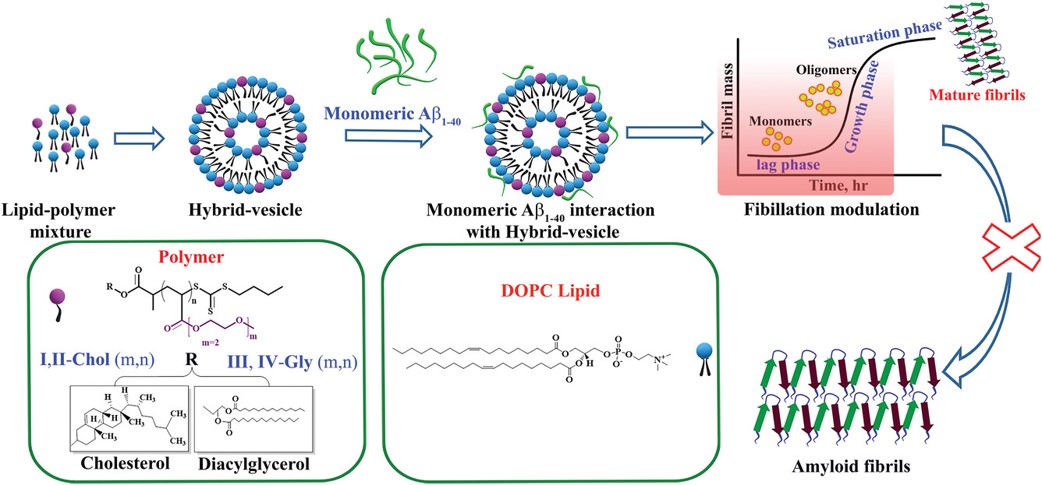
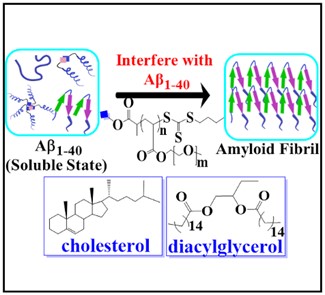
Solubility and aggregation of proteins are crucial factors for their functional and further biological roles. Aggregation of proteins in vivo, such as the amyloid beta (Aβ1–40) peptide into fibrils, is significantly modulated by membrane lipids, abundantly present in cells. We developed a model membrane system, composed of lipid hybrid-vesicles bearing embedded hydrophilic polymers to in vitro study the aggregation of the Aβ1–40 peptide. Focus is to understand and inhibit the primordial, nucleation stages of their fibrillation by added hybrid-vesicles, composed of a natural lipid and amphiphilic polymers. These designed hybrid-vesicles are based on 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), displaying embedded hydrophilic (EO)mPnA_EG polymers (m = 2 or 3; Pn = 10 to 52 with Mn = 2800–9950 gmol−1) in amounts ranging from 5–20 mol%, anchored to the POPC vesicles via hydrophobic hexadecyl-, glyceryl- and cholesteryl-moieties, affixed to the polymers as end-groups. All investigated hybrid-vesicles significantly delay fibrillation of the Aβ1–40 peptide as determined by thioflavin T (ThT) assays. We observed that the hybrid-vesicles interacted with early aggregating species of Aβ1–40 peptide, irrespective of their composition or size. A substantial perturbation of both primary (k+kn) and secondary (k+k2) nucleation rates of Aβ1–40 by the POPC–polymer vesicles compared to POPC vesicles was observed, particularly for the cholesteryl-anchored polymers, interfering with the fragmentation and elongation steps of Aβ1–40.. Reproduced with permission from the Royal Society of Chemistry.