Multisegmented Hybrid Polymer Based on Oligo-Amino Acids: Synthesis and Secondary Structure in Solution and in the Solid State.
Freudenberg, J., et al. Macromolecules 2019,52 (12), 4534-4544,DOI: https://doi.org/10.1021/acs.macromol.9b00684.
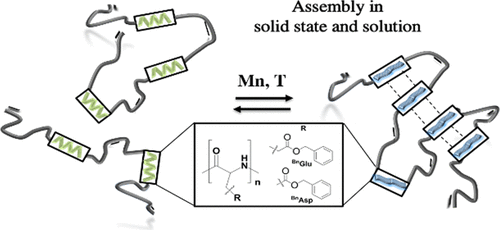
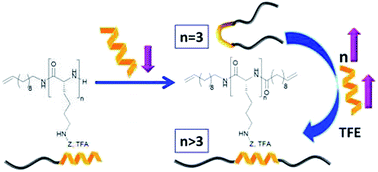
The synthesis of multisegmented polymers containing repetitive oligo-(BnAspn, BnGlun) sequences separated by methylene units and their secondary structure formation in solution and in the solid state are reported. Combining ring-opening polymerization (ROP) of N-carboxyanhydrides with postfunctionalization of the respective N-terminus yields the oligo-( BnAsp3, BnAsp10, BnGlu3, and BnGlu10) sequences bearing terminal vinyl groups on either side of the polymer chain by preparative gel permeation chromatography generate polymers of a precise degree of polymerization (n = 3, 10; m = 1–136). β-sheet conformation is identified as the thermodynamically more stable conformation, as supported by our experiments in the solid state and in solution. Reproduced with permission. Copyright 2019©, American Chemical Society.