Chemical Structure-Dependence of the Thermal Transition in Thermoresponsive Ethylene-Glycol-Containing Polymers as Characterized by EPR Spectroscopy
Haeri, H. H. et. al., Macromol. Chem. Phys. 2025, 226, 10, 2500050, https://doi.org/10.1002/macp.202500050
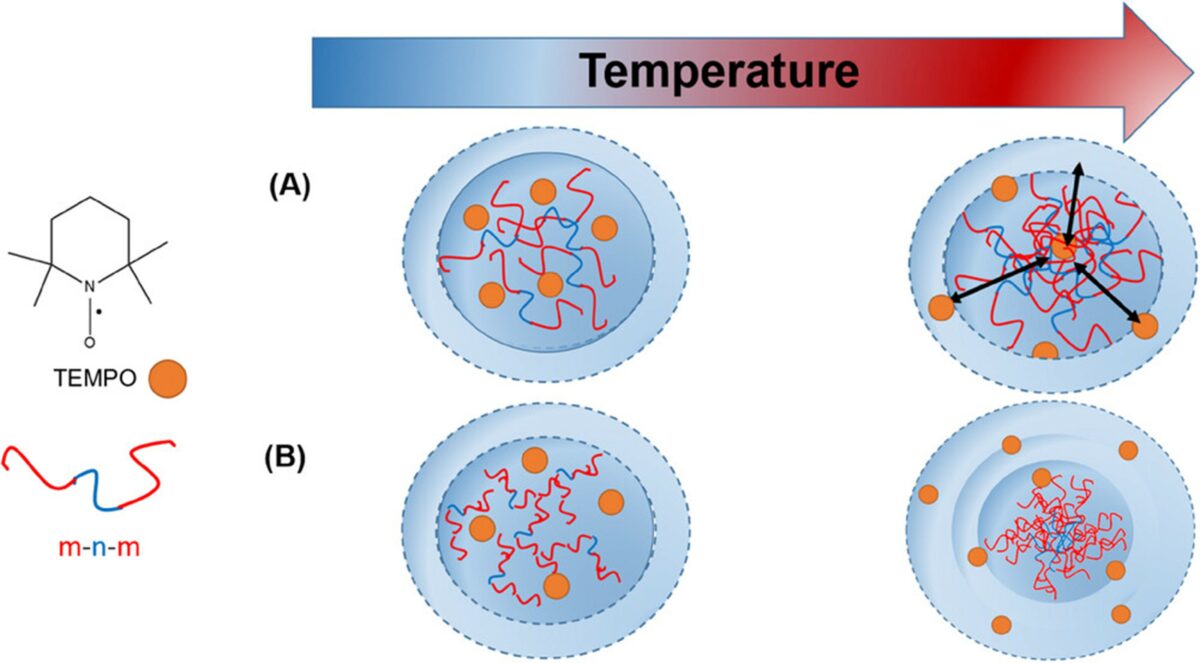
Here, the phase transition behavior of thermoresponsive poly(oligo(ethylene glycol)acrylates) is systematically examined with respect to these three factors. Spin-probing electron paramagnetic resonance (EPR) spectroscopy is employed to investigate the impact of these parameters below and at the macroscopically observable thermal transition, as well as to characterize the nanoscale inhomogeneities associated with the transition. This study finds that the (de)hydration behavior of the polymer chains is mainly determined by the length of ethylene glycol side chains but the individual single polymer end groups also have remarkable influence on the collapse behavior. © 2025 The Author(s). Macromolecular Chemistry and Physics published by Wiley‐VCH GmbH. Open access