One traditional focus of our research group is directed to novel polymers, novel monomers and novel synthetic methodologies. Principles of organic synthesis, macromolecular chemistry and catalyst-design are addressed, aiming to precisely engineer a macromolecules’ chemical identity.1, 2-6 To this endeavor precise chain length, low polydispersities, and the desired composition and arrangement of monomers are embedded into the desired architecture. Therefore a major focus is directed on living polymerization methods, where significant effort is placed on living carbocationic polymerization (LCCP), RAFT-polymerization, nitroxide mediated polymerization (NMP), living ring-opening polymerization (ROP), ring-opening metathesis polymerization (ROMP) and anionic polymerization (LAP). Besides endgroup- and side-chain modifications of synthetic polymers and proteins, effort has been placed for “click”-based methodologies in polymer science.
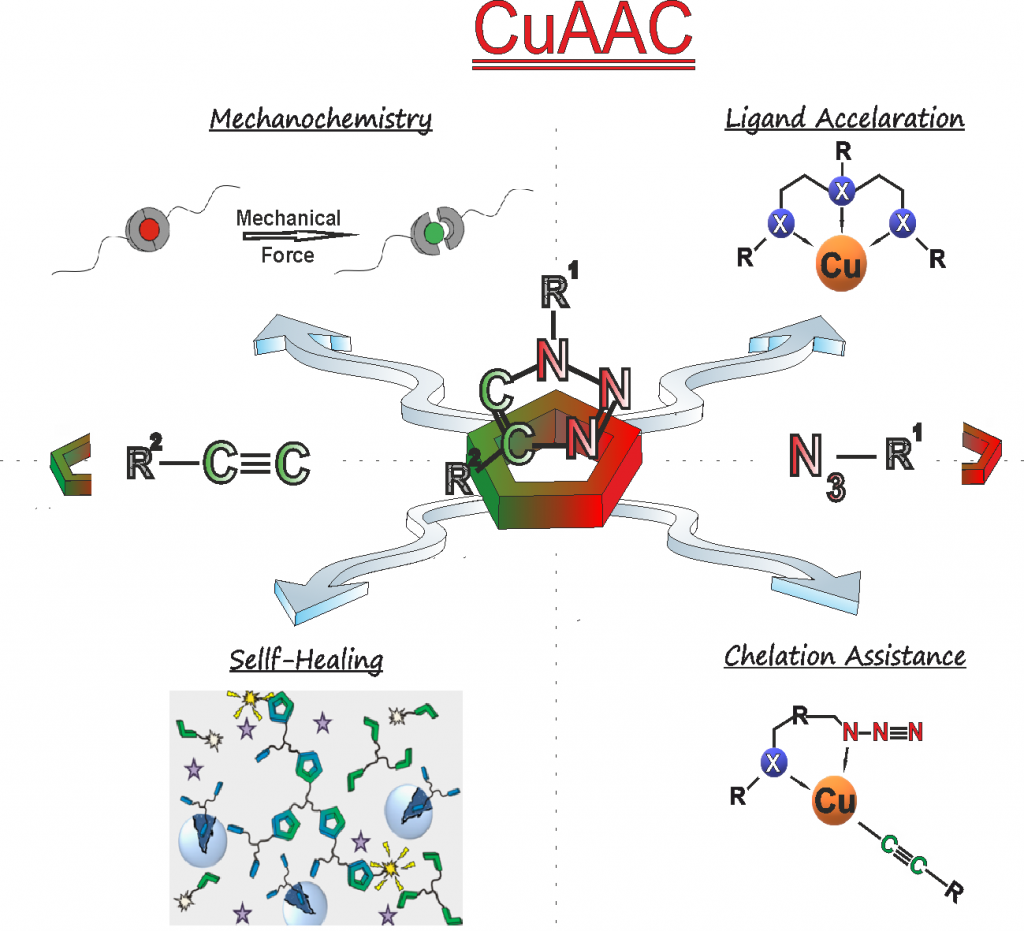
Being among the first ever in 20047 to apply CuAAc (copper catalyzed “click”-chemistry) in combination with living polymerization (see our first landmark paper in this field) we have developed and used CuAAc extensively during the past decades (see our selected reviews from 20075, 20084 and 20192 as examples). Based on its extremely high functional group tolerance, its robustness due to autoacceleration-effects8, 9 and chelation-assistance,10 CuAAc is currently the only truly useful click-chemistry in Barry Sharpless definition, widely applied in polymer science. It is this knowledge which ensures fast, efficient, and also reliable transformations before, during and even after polymerization. Novel developments of our research group deal with the “thio-bromo-click”-chemistry”11-13, being advantageous if biomolecules and the conjugation of polymers onto biomolecules is desired.13 Significant effort is spent to generate biohybrid-molecules, where polymer science and proteins/peptides meet, may it be as polymer-peptide-conjugates,12, 13 as polymer-protein-conjugates,14, 15 or as artificial beta-turn-mimetics.16, 17 Recent publications show the expansion of CuAAc to mechanochemically induced chemistries.18
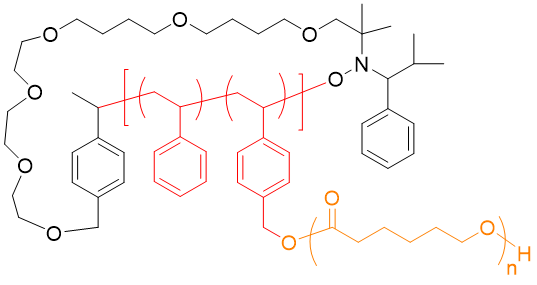
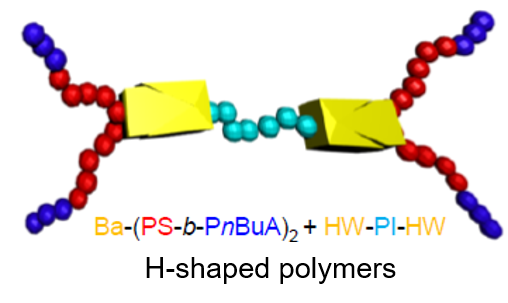
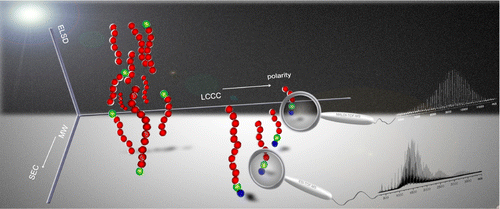
The synthesis of complex supramolecular architectures, able to assemble via hydrogen bonds is a longlasting topic in our group. Based on early work, where end-group-modified polymers were synthesized via LCCP-chemistry,19 the approaching RAFT-, ROP-, ADMET, and ROMP-polymerization-methods have allowed to prepare highly complex polymer architectures. It must be mentioned that now, in combination with CuAAC and “thiobromo-click”-chemistry,12nearly any functional group can be connected onto a desired polymer backbone. Recent examples are related to combinations of RAFT-polymerization bearing complex hydrogen bonds20-24, 25 and ionomers,26 ROMP-6, 7, 27, 28, combinations of ROP (N-carboxyanhydrides, 1,3-oxazolines, caprolactones/caprolactames)17, 29-33 with/without ADMET30, 34-36, NMP10, 8, 37. Only the proper combination of high-resolution mass spectrometry (ESI/MALDI-TOF), often combined with liquid chromatography is then able to proof purity of these samples and resolve their precise structure. Coupled and hyphenated techniques have been developed to this endeavor in our group (ESI/MALDI-TOF – 2D-chromatrography)38 to achieve information on complex architecture and substitution patterns or crossover-chemistries.39 Recent work focusses on complex supramolecular polymers with defined positioning of H-bonding and halogen-N interactions.20, 22, 30, 40
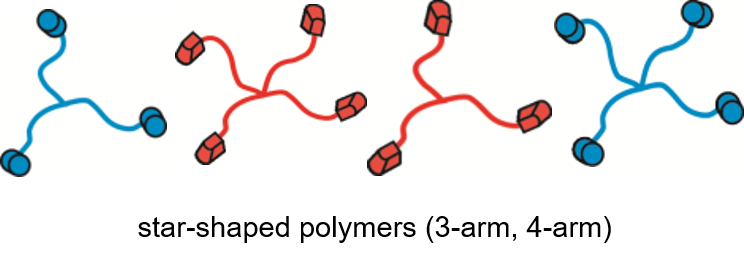
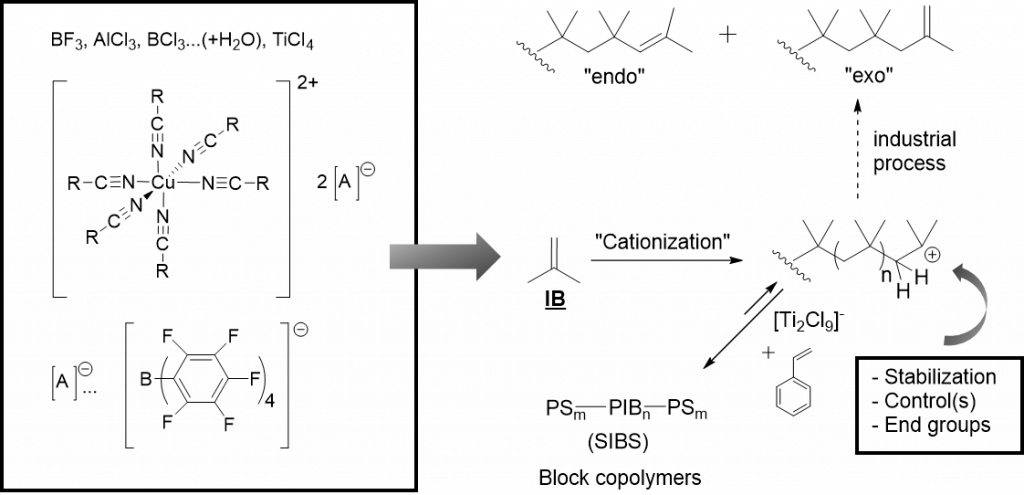
There has been – and still is – a longlasting tradition in living carbocationic polymerization (LCCP) in our research-group, where polyisobutylene (PIB) is prepared by living carbocationic polymerization (LCCP). PIB is one of the few and only polymers only addressable by cationic polymerization. Being one of the few truly biocompatible polymers, defined architectures of PIB-polymers (linear-19, block-41, star-,10, 41-43 endgroup-functionalized44-45, sidegroup-functionalized46, hyperbranched-,47 cyclic48, grafting-from-SiO249) are addressed in our group via LCCP, used for subsequent (biomimetic) materials16, 50-53, self-healing elastomers21, 45, 54, 55and ionic liquids56-57. We are further developing novel PIB-polymers for advanced applications in biomedicine and technology, as biocompatible polymers, surfaces, and as self-healing materials.Recently we are exploiting PIB as 3D-printable polymer and as slow-release system for drugs.58, 59
References
- Binder, W. H., et al., “Click”-Chemistry in Macromolecular Synthesis. In Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc: 2009; DOI:https://doi.org/10.1002/0471440264.pst565. Binder, W. H., et al., “Click”-chemistry on supramolecular materials. In Click Chemistry for Biotechnology and Materials Science, Lahann, J., Ed. Wiley-Blackwell: 2009; pp 119-175, DOI:https://doi.org/10.1002/9780470748862.ch7. Kurzhals, S., et al., Combination of Olefin Metathesis Polymerization with Click Chemistry. In Handbook of Metathesis, Edition: 2nd, Robert H. Grubbs, E. K., Ed. John Wiley & Sons: 2015; pp 207-227, DOI:https://doi.org/10.1002/9783527674107.ch35.
- Neumann, S., et al., The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromolecular Rapid Communications 2019, 1900359, DOI:https://doi.org/10.1002/marc.201900359.
- Shaygan Nia, A., et al., Graphene as initiator/catalyst in polymerization chemistry. Progress in Polymer Science 2017, 67, 48-76, DOI:http://dx.doi.org/10.1016/j.progpolymsci.2016.12.005.
- Binder, W. H., et al., “Click”-Chemistry in Polymer and Material Science: An Update. Macromolecular Rapid Communications 2008, 29, 952-981, DOI:http://dx.doi.org/10.1002/marc.200800089.
- Binder, W. H., et al., “Click” Chemistry in Polymer and Materials Science. Macromolecular Rapid Communications 2007, 28 (1), 15-54, DOI:https://doi.org/10.1002/marc.200600625.
- Kurzhals, S., et al., Combination of Olefin Metathesis Polymerization with Click Chemistry. In Handbook of Metathesis, Edition: 2nd, Robert H. Grubbs, E. K., Ed. John Wiley & Sons: 2015; pp 207-227, DOI:https://doi.org/10.1002/9783527674107.ch35.
- Binder, W. H., et al., Combining Ring-Opening Metathesis Polymerization (ROMP) with Sharpless-Type “Click” Reactions: An Easy Method for the Preparation of Side Chain Functionalized Poly(oxynorbornenes). Macromolecules 2004, 37 (25), 9321-9330, DOI:http://dx.doi.org/10.1021/ma0480087.
- Döhler, D., et al., Autocatalysis in the Room Temperature Copper(I)-Catalyzed Alkyne–Azide “Click” Cycloaddition of Multivalent Poly(acrylate)s and Poly(isobutylene)s. Macromolecules 2012, 45 (8), 3335-3345, DOI:http://dx.doi.org/10.1021/ma300405v.
- Kargarfard, N., et al., Improving Kinetics of “Click-Crosslinking” for Self-Healing Nanocomposites by Graphene-Supported Cu-Nanoparticles. Polymers 2018, 10 (1), 17, DOI: https://doi.org/10.3390/polym10010017.
- Neumann, S., et al., Chelation-assisted CuAAC in star-shaped polymers enables fast self-healing at low temperatures. Polymer Chemistry 2016, 7 (13), 2342-2351, DOI:http://dx.doi.org/10.1039/C5PY01818H.
- Chen, S., et al., Orthogonal Modification of Polymers via Thio–Bromo “Click” Reaction and Supramolecular Chemistry: An Easy Method Toward Head-to-Tail Self-Assembled Supramolecular Polymers. ACS Macro Letters 2015, 4, 48-52, DOI:http://dx.doi.org/10.1021/mz500747t.
- Kumar, S., et al., Thio-Bromo “Click” Reaction Derived Polymer–Peptide Conjugates for Their Self-Assembled Fibrillar Nanostructures. Macromolecular Bioscience 2020, 2000048, DOI:https://doi.org/10.1002/mabi.202000048.
- Kumar, S., et al., One-Pot Synthesis of Thermoresponsive Amyloidogenic Peptide–Polymer Conjugates via Thio–Bromo “Click” Reaction of RAFT Polymers. Macromolecular Rapid Communications 2017, 1700507, DOI:http://dx.doi.org/10.1002/marc.201700507.
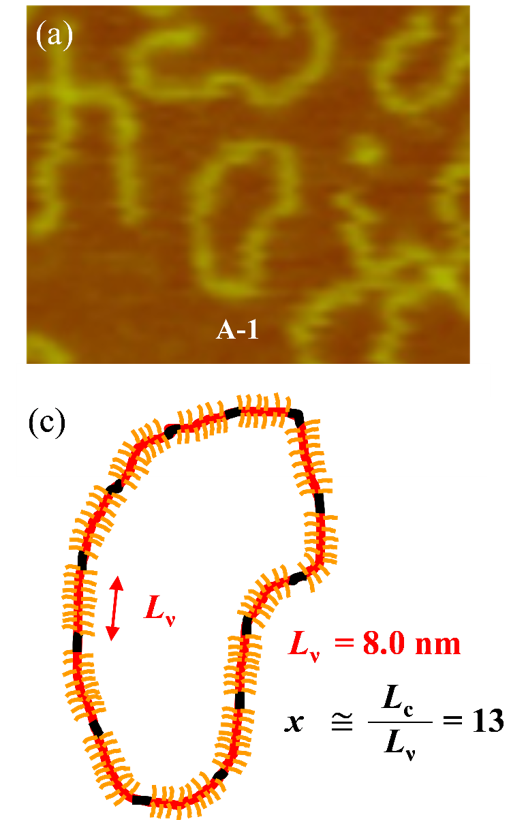
- Evgrafova, Z., et al., Probing Polymer Chain Conformation and Fibril Formation of Peptide Conjugates. ChemPhysChem 2019, 20 (2), 236-240, DOI:https://doi.org/10.1002/cphc.201800867.
- Evgrafova, Z., et al., Synthesis and Aggregation of Polymer-Amyloid β Conjugates. Macromolecular Rapid Communications 2019, 1900378, DOI:https://doi.org/10.1002/marc.201900378.
- Malke, M., et al., Synthesis of an Amphiphilic β-Turn Mimetic Polymer Conjugate. ACS Macro Letters 2014, 3 (4), 393-397, DOI:http://pubs.acs.org/doi/abs/10.1021/mz500108n.
- Deike, S., et al., Induction of Chirality in β-Turn Mimetic Polymer Conjugates via Postpolymerization “Click” Coupling. Macromolecules 2017, 50 (7), 2637-2644, DOI:http://dx.doi.org/10.1021/acs.macromol.7b00343.
- Adam, C., et al., The Role of Terminal Functionality in the Membrane and Antibacterial Activity of Peptaibol-Mimetic Aib Foldamers. Chemistry-a European Journal 2018, 24 (9), 2249-2256, DOI:https://doi.org/10.1002/chem.201705299.
- Binder, W. H., et al., Tunable Materials from Hydrogen-Bonded Pseudo Block Copolymers. Advanced Materials 2005, 17 (23), 2824-2828, DOI:http://dx.doi.org/10.1002/adma.200501505. Kunz, M. J., et al., Hydrogen-bonded supramolecular poly(ether ketone)s. Journal of Polymer Science Part A: Polymer Chemistry 2004, 42 (3), 661-674, DOI:http://dx.doi.org/10.1002/pola.10858. Binder, W. H., et al., Synthesis and Analysis of Telechelic Polyisobutylenes for Hydrogen-Bonded Supramolecular Pseudo-Block Copolymers. Macromolecules 2004, 37 (5), 1749-1759, DOI:http://dx.doi.org/10.1021/ma034924t.
- Chen, S., et al., Opposing Phase-Segregation and Hydrogen-Bonding Forces in Supramolecular Polymers. Angewandte Chemie International Edition 2017, 56 (42), 13016-13020, DOI:http://dx.doi.org/10.1002/anie.201707363.
- Callies, X., et al., Effects of multifunctional cross-linkers on rheology and adhesion of soft nanostructured materials. Soft Matter 2017, 13 (43), 7979-7990, DOI:http://dx.doi.org/10.1039/C7SM01304C.
- Chen, S., et al., Hierarchical Micelles via Polyphilic Interactions: Hydrogen-Bonded Supramolecular Dendrons and Double Immiscible Polymers. Nano Letters 2016, 16 (2), 1491-1496, DOI:http://dx.doi.org/10.1021/acs.nanolett.5b05203.
- Chen, S., et al., Rheology of hydrogen-bonded dendritic supramolecular polymer networks in the melt state. Polymer 2016, 107, 466-473, DOI:http://dx.doi.org/10.1016/j.polymer.2016.08.046.
- Chen, S., et al., One-pot synthesis and self-assembly of supramolecular dendritic polymers. Polymer Chemistry 2015, 6 (46), 7988-7994, DOI:http://dx.doi.org/10.1039/C5PY01329A. Chen, S., et al., Controlled copolymerization of n-butyl acrylate with semifluorinated acrylates by RAFT polymerization. Polym. Chem. 2015, 6 (3), 448-458, DOI:https://doi.org/10.1039/C4PY01084A. Chen, S., et al., Facile preparation of supramolecular (ABAC)n multiblock copolymers from Hamilton wedge and barbiturate-functionalized RAFT agents. Polymer Chemistry 2014, 5 (8), 2891-2900, DOI:http://dx.doi.org/10.1039/C3PY01482G.
- Chen, S., et al., Engineering the Morphology of Hydrogen-Bonded Comb-Shaped Supramolecular Polymers: From Solution Self-Assembly to Confined Assembly. Polymer Chemistry 2020, DOI:http://dx.doi.org/10.1039/D0PY00570C.
- Chen, S., et al., Synthesis and Morphology of Semifluorinated Polymeric Ionic Liquids. Macromolecules 2018, 51 (21), 8620-8628, DOI:https://doi.org/10.1021/acs.macromol.8b01624. Chen, S., et al., Gating effects of conductive polymeric ionic liquids. Journal of Materials Chemistry C 2018, 6 (30), 8242-8250, DOI:http://dx.doi.org/10.1039/C8TC01936C.
- Pulamagatta, B., et al., Shear induced structure orientation in norbornene block copolymers: In-situ Rheo-SAXS investigations. European Polymer Journal 2012, 48 (0), 1127-1134, DOI:https://doi.org/10.1016/j.eurpolymj.2012.03.017. Binder, W. H., et al., Synthesis and Self-Assembly of Hydrogen-Bonded Supramolecular Polymers. In Complex Macromolecular Architectures, John Wiley & Sons (Asia) Pte Ltd: 2011; pp 53-95, DOI:http://dx.doi.org/10.1002/9780470825150.ch3. Olubummo, A., et al., Synthesis and crossover reaction of TEMPO containing block copolymer via ROMP. Beilstein Journal of Organic Chemistry 2010, 6 (59),DOI:doi:10.3762/bjoc.6.59. Kurzhals, S., et al., Telechelic polynorbornenes with hydrogen bonding moieties by direct end capping of living chains. Journal of Polymer Science Part A: Polymer Chemistry 2010, 48 (23), 5522-5532, DOI:http://dx.doi.org/10.1002/pola.24362. Kluger, C., et al., Functionalized poly(oxanorbornene)-block-copolymers: Preparation via ROMP/click-methodology. Journal of Polymer Science Part A: Polymer Chemistry 2007, 45 (3), 485-499, DOI:http://dx.doi.org/10.1002/pola.21867. Binder, W. H., et al., Directing Supramolecular Nanoparticle Binding onto Polymer Films: Film Formation and Influence of Receptor Density on Binding Densities. Macromolecules 2006, 39 (23), 8092-8101, DOI:http://dx.doi.org/10.1021/ma061256d. Binder, W. H., et al., Directed Nanoparticle Binding onto Microphase-Separated Block Copolymer Thin Films. Macromolecules 2005, 38 (23), 9405-9410, DOI:http://dx.doi.org/10.1021/ma0518252. Binder, W. H., et al., Homologous Poly(isobutylene)s: Poly(isobutylene)/High-Density Poly(ethylene) Hybrid Polymers. Macromolecules 2008, 41 (22), 8405-8412, DOI:http://pubs.acs.org/doi/abs/10.1021/ma801465r.
- Binder, W. H.; Enders, C.; Herbst, F.; Hackethal, K. Synthesis and Self-Assembly of Hydrogen-Bonded Supramolecular Polymers. In Complex Macromolecular Architectures, John Wiley & Sons (Asia) Pte Ltd, 2011; pp 53-95, DOI:https://doi.org/10.1002/9780470825150.ch3.
- Freudenberg, J., et al., Chirality Control of Screw-Sense in Aib-Polymers: Synthesis and Helicity of Amino Acid Functionalized Polymers. ACS Macro Letters 2020, 686-692, DOI:https://doi.org/10.1021/acsmacrolett.0c00218.
- Freudenberg, J., et al., Multisegmented Hybrid Polymer Based on Oligo-Amino Acids: Synthesis and Secondary Structure in Solution and in the Solid State. Macromolecules 2019, 52 (12), 4534-4544, DOI:https://doi.org/10.1021/acs.macromol.9b00684.
- Canalp, M. B., et al., Secondary structure of end group functionalized oligomeric-l-lysines: investigations of solvent and structure dependent helicity. RSC Advances 2019, 9 (38), 21707-21714, DOI:http://dx.doi.org/10.1039/C9RA03099A.
- Einzmann, M., et al., Novel functional initiators for oxazoline polymerization. Journal of Polymer Science Part A: Polymer Chemistry 2001, 39 (16), 2821-2831, DOI:http://dx.doi.org/10.1002/pola.1262. Binder, W. H., et al., Block copolymers derived from photoreactive 2-oxazolines, 1. Synthesis and micellization behavior. Macromolecular Chemistry and Physics 2000, 201 (9), 949-957.
- Ostas, E., et al., Crystallization of Supramolecular Pseudoblock Copolymers. Macromolecules 2013, 46 (11), 4481-4490, DOI:http://dx.doi.org/10.1021/ma400622w. Ostas, E., et al., Poly(ε-caprolactone)–poly(isobutylene): A crystallizing, hydrogen-bonded pseudo-block copolymer. Journal of Polymer Science Part A: Polymer Chemistry 2011, 49 (15), 3404-3416, DOI:http://dx.doi.org/10.1002/pola.24777.
- Freudenberg, J., et al., Precision polymers containing main-chain-amino acids: ADMET polymerization and crystallization. RSC Advances 2017, 7 (75), 47507-47519, DOI:http://dx.doi.org/10.1039/C7RA10485E.
- Canalp, M. B., et al., Hybrid polymers bearing oligo-l-lysine(carboxybenzyl)s: synthesis and investigations of secondary structure. RSC Advances 2020, 10 (3), 1287-1295, DOI:http://dx.doi.org/10.1039/C9RA09189K.
- Appiah, C., et al., Crystallization behavior of precision polymers containing azobenzene defects. European Polymer Journal 2017, 97 (Supplement C), 299-307, DOI:https://doi.org/10.1016/j.eurpolymj.2017.10.023. Appiah, C., et al., Synthesis of photoresponsive main-chain oligomers with azobenzene moieties via ADMET oligomerization and their micellization properties. Polymer Chemistry 2017, 8 (18), 2752-2763, DOI:http://dx.doi.org/10.1039/C7PY00426E. Appiah, C., et al., Synthesis and characterization of new photoswitchable azobenzene-containing poly(?-caprolactones). RSC Advances 2016, 6 (8), 6358-6367, DOI:http://dx.doi.org/10.1039/C5RA25216D.
- Narumi, A., et al., Evaluation of Ring Expansion-Controlled Radical Polymerization System by AFM Observation. ACS Macro Letters 2019, 8 (6), 634-638, DOI:https://doi.org/10.1021/acsmacrolett.9b00308. Narumi, A., et al., Cyclic alkoxyamine-initiator tethered by azide/alkyne-“click”-chemistry enabling ring-expansion vinyl polymerization providing macrocyclic polymers. Journal of Polymer Science Part A: Polymer Chemistry 2010, 48 (15), 3402-3416, DOI:http://dx.doi.org/10.1002/pola.24126. Binder, W. H., et al., Telechelic Poly(N-isopropylacrylamides) via Nitroxide-Mediated Controlled Polymerization and “Click” Chemistry: Livingness and “Grafting-from” Methodology. Macromolecules 2007, 40 (9), 3097-3107, DOI:http://dx.doi.org/10.1021/ma0628376.
- Barqawi, H., et al., 2D-LC/SEC-(MALDI-TOF)-MS Characterization of Symmetric and Nonsymmetric Biocompatible PEOm–PIB–PEOn Block Copolymers. Macromolecules 2013, 46 (19), 7638-7649, DOI:http://dx.doi.org/10.1021/ma401604h. Barqawi, H., et al., Multidimensional Characterization of α,ω-Telechelic Poly(ε-caprolactone)s via Online Coupling of 2D Chromatographic Methods (LC/SEC) and ESI-TOF/MALDI-TOF-MS. Macromolecules 2012, 45 (24), 9779–9790, DOI:http://dx.doi.org/10.1021/ma3016739.
- Kurzhals, S., et al., Monitoring ROMP Crossover Chemistry via ESI-TOF MS. Macromolecules 2013, 46 (3), 597-607, DOI:http://dx.doi.org/10.1021/ma302555q. Binder, W. H., et al., Monitoring Block-Copolymer Crossover-Chemistry in ROMP: Catalyst Evaluation via Mass-Spectrometry (MALDI). Macromolecules 2009, 42 (24), 9457-9466, DOI:http://dx.doi.org/10.1021/ma902115j.
- Chen, S., et al., Hydrogen-Bonded Supramolecular Polymer Adhesives: Straightforward Synthesis and Strong Substrate Interaction, Angewandte Chemie International Edition, 2022, 61, e202203876, DOI:https://doi.org/10.1002/anie.202203876. Zheng, X., et al., Halogen-Bond Mediated 3D Confined Assembly of AB Diblock Copolymer and C Homopolymer Blends, Small 2021, 2007570, DOI:https://doi.org/10.1002/smll.202007570. Döhler, D., et al., Supramolecular H-bonded three-arm star polymers by efficient combination of RAFT polymerization and thio-bromo “click” reaction, Polymer, 2017, 122, 148-158, DOI:https://doi.org/10.1016/j.polymer.2017.06.067.
- Fuchs, C., et al., Molecular arrangement of symmetric and non-symmetric triblock copolymers of poly(ethylene oxide) and poly(isobutylene) at the air/water interface. Journal of Colloid and Interface Science 2015, 437 (0), 80-89, DOI:http://dx.doi.org/10.1016/j.jcis.2014.09.050.
- Rother, M., et al., Synthesis and Organization of Three-Arm-Star PIB-PEO Block Copolymers at the Air/Water Interface: Langmuir- and Langmuir-Blodgett Film Investigations. Macromolecular Chemistry and Physics 2010, 211 (2), 204-214, DOI:http://dx.doi.org/10.1002/macp.200900435.
- Rupp, H., et al., 3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromolecular Rapid Communications 2019, 1900467, DOI:https://doi.org/10.1002/marc.201900467.
- Binder, W. H., et al., Poly(ether ketone)-polyisobutylene block copolymers: Synthesis and phase behavior. Journal of Polymer Science Part A: Polymer Chemistry 2005, 43 (1), 188-202, DOI:http://dx.doi.org/10.1002/pola.20483.
- Herbst, F., et al., Aggregation and chain dynamics in supramolecular polymers by dynamic rheology: cluster formation and self aggregation. Macromolecules 2010, 43, 10006–10016, DOI:http://dx.doi.org/10.1021/ma101962y.
- Hackethal, K., et al., Introducing Polar Monomers into Polyisobutylene by Living Cationic Polymerization: Structural and Kinetic Effects. Macromolecules 2010, 43 (4), 1761-1770, DOI:http://dx.doi.org/10.1021/ma9025114.
- Döhler, D., et al., Hyperbranched polyisobutylenes for self-healing polymers. Polymer Chemistry 2014, 5 (3), 992-1000, DOI:http://dx.doi.org/10.1039/C3PY01151H.
- Schulz, M., et al., Macrocyclization of polymers via ring-closing metathesis and azide/alkyne-“click”-reactions: An approach to cyclic polyisobutylenes. Journal of Polymer Science Part A: Polymer Chemistry 2010, 48 (3), 671-680, DOI:http://dx.doi.org/10.1002/pola.23820.
- Binder, W. H., et al., Grafting Polyisobutylene from Nanoparticle Surfaces: Concentration and Surface Effects on Livingness. Macromolecules 2009, 2 (19), 7379-7387, DOI:http://pubs.acs.org/doi/abs/10.1021/ma900535e.
- Schulz, M., et al., Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromolecular Rapid Communications 2015, 36 (23), 2031-2041, DOI:http://dx.doi.org/10.1002/marc.201500344.
- Schulz, M., et al., Beyond the Lipid-Bilayer: Interaction of Polymers and Nanoparticles with Membranes. Soft Matter 2012, 8 (18), 4849–4864, DOI:http://dx.doi.org/10.1039/C2SM06999G. Olubummo, A., et al., Phase Changes in Mixed Lipid/Polymer Membranes by Multivalent Nanoparticle Recognition. Langmuir 2014, 30 (1), 259-267, DOI:http://dx.doi.org/10.1021/la403763v. Schulz, M., et al., Hybrid lipid/polymer giant unilamellar vesicles: effects of incorporated biocompatible PIB-PEO block copolymers on vesicle properties. Soft Matter 2011, 7 (18), 8100-8110, DOI:http://dx.doi.org/10.1039/C1SM05725A.
- Schulz, M., et al., Controlling Molecular Recognition with Lipid/Polymer Domains in Vesicle Membranes. Angewandte Chemie International Edition 2013, 52 (6), 1829-1833, DOI:http://dx.doi.org/10.1002/anie.201204959.
- Olubummo, A., et al., Controlling the Localization of Polymer-Functionalized Nanoparticles in Mixed Lipid/Polymer Membranes. ACS Nano 2012, 6 (10), 8713–8727, DOI:http://dx.doi.org/10.1021/nn3023602.
- Yan, T., et al., Unveiling the molecular mechanism of self-healing in a telechelic, supramolecular polymer network. Scientific Reports 2016, 6, 32356, DOI:https://doi.org/10.1038/srep32356.
- Yan, T., et al., Nanostructure and Rheology of Hydrogen-Bonding Telechelic Polymers in the Melt: From Micellar Liquids and Solids to Supramolecular Gels. Macromolecules 2014, 47 (6), 2122-2130, DOI:http://dx.doi.org/10.1021/ma402007f.
- Frenzel, F., et al., Molecular Dynamics and Charge Transport in Polymeric Polyisobutylene-Based Ionic Liquids. Macromolecules 2016, 49 (7), 2868-2875, DOI:http://dx.doi.org/10.1021/acs.macromol.6b00011.
- Stojanovic, A., et al., Designing melt flow of poly(isobutylene)-based ionic liquids. Journal of Materials Chemistry A 2013, 1 (39), 12159-12169, DOI:http://dx.doi.org/10.1039/C3TA12646C.
- Hilgeroth, P.S., et al., 3D-printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene-Isobutylene-Styrene as a Slow Release System, Polymers, 2022, 14, 3742, DOI:https://doi.org/10.3390/polym14183742. Du, F., et al., 3D-printing of the polymer/insect-repellent system poly(l-lactic acid)/ethyl butylacetylaminopropionate (PLLA/IR3535). International Journal of Pharmaceutics 2022, 624, 122023, DOI:https://doi.org/10.1016/j.ijpharm.2022.122023. Rupp, H., et al.,3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromolecular Rapid Communications 2019, 40 (24), 1900467, DOI:https://doi.org/10.1002/marc.201900467.
- Rupp, H., et al., 3D Printing of Solvent-Free Supramolecular Polymers. Frontiers in Chemistry 2021, 9, 771974, DOI:https://doi.org/10.3389/fchem.2021.771974.