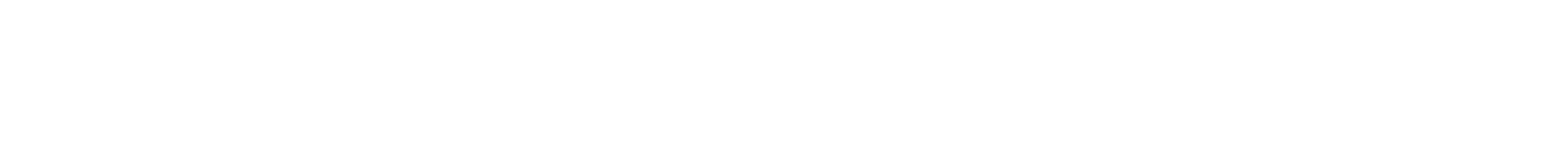Secondary-structure of proteins and peptides is crucial for their function in living systems, in its undesired assembly being responsible for e. g. Alzheimer’s or Parkinsons’ disease.1 Within the DFG-funded collaborative research project SFB TRR 102 two projects deal with amyloid-proteins to understand and prevent disease-relevant aggregation via synthetic polymers, able to fold- and unfold during aggregation. Main aim is to understand, control, and inhibit fibrillation by concepts of polymer-science and a deeper understanding of the fibrillation processes. Concepts of synthetic chemistry are combined with proteins to generate hybrid-systems, able to fold and aggregate similar to the native peptide systems. Both, peptide- and polymer chemistry are researched in this area.
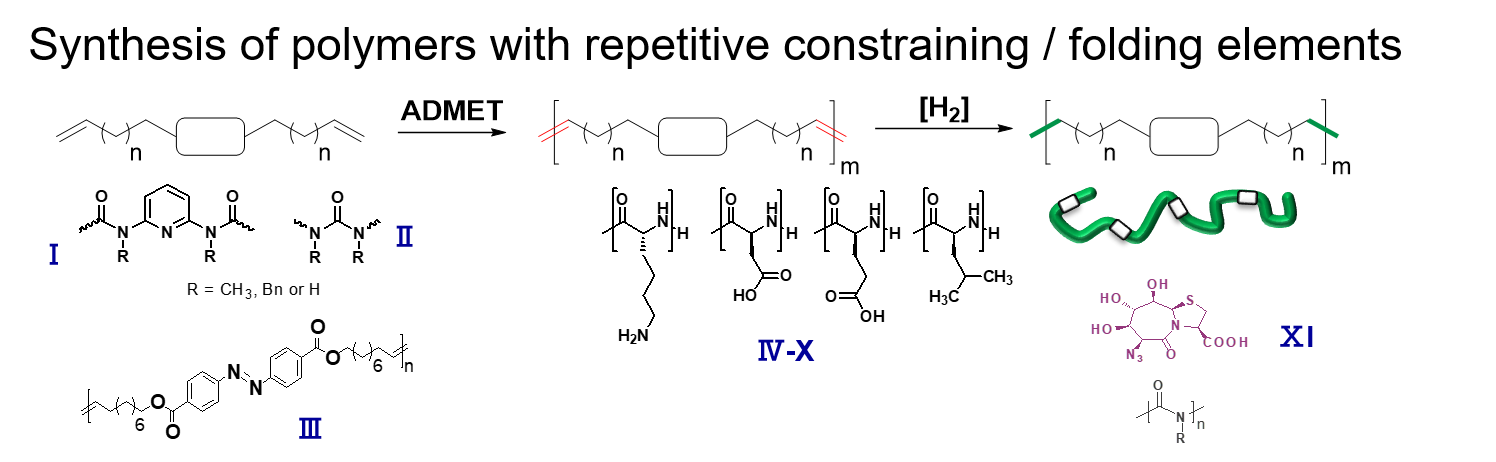
Synthesis via ring-opening (ROP) and ADMET-polymerization generates precision-hybrid-polymers consisting of polyamino-acids and repetitive middle segments displaying a defined flexibility/rigidity.2, 3 They allow to study refolding and cooperative phenomena, similar to protein folding and amyloid assembly.4-7 Recent activities are directed to understand the influence of distance, endgroups and numbers of the folding elements on the final three-dimensional structure of the assemblies. Photoswitchability,8 assembly with embedded constrained folding elements,2 and dynamic secondary structure-elements (such as beta-folds, alpha-helices)4, 6 were successfully introduced into the polymer chains, acting as biomimetics for larger proteins.4-7 Currently, the concept is extended onto polymer/amyloid-molecules to design, understand and influence the folding and aggregation pathways of new such detrimentally aggregating proteins.9, 10
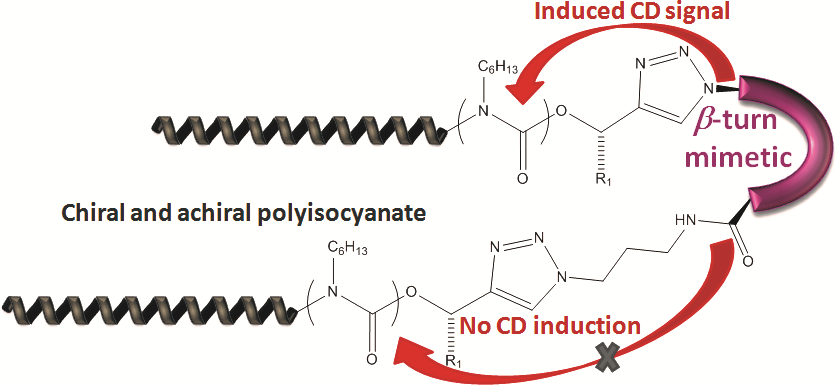
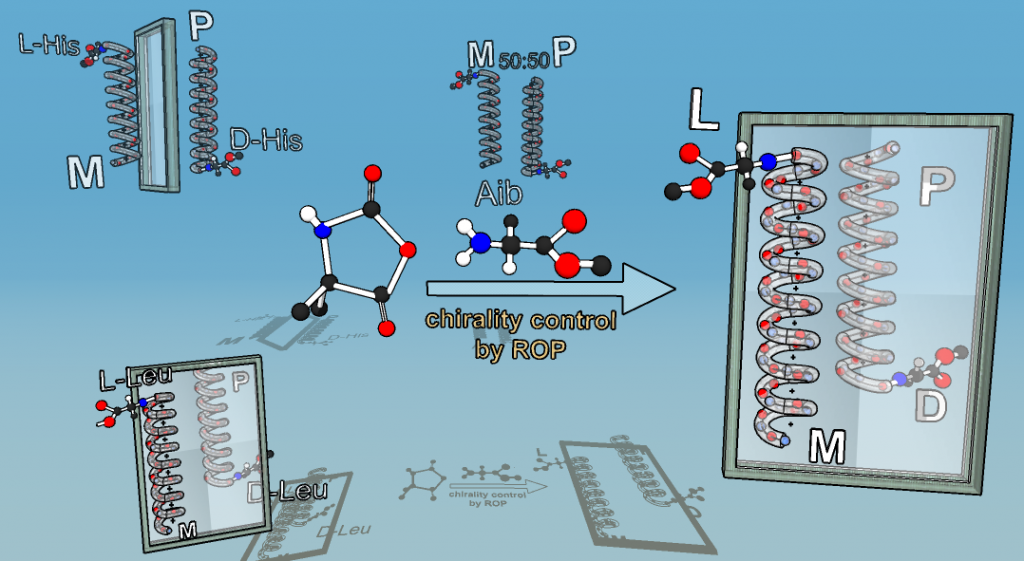
Chirality is among the chemical principles required for life. We generate molecules with switchable chirality, where the transfer of chirality onto nonchiral side-chains is probed. As probed in helically chiral polymers,11, 12 the transfer of only one chiral group can have influence on many tens of monomers, linked into a polymer chain to yield either right- or left-handed helices, induced just by one chiral moiety.13 We employ precisely engineered polymers based on achiral polyisocyanides, polyisocyanates and achiral polyaminoacids to study the influence of a singular chiral-element embedded in the polymer chain. It demonstrates that only one single chiral moiety can display large chirality-transfer-effects, also termed chiral amplification, taken place presumptively in the early times of the universe. Recent work describes the effects of various head-groups together with an expansion towards induction of chirality in the solid state and the use of chiral induction in transistors.14
References
- Binder, W. H., et al., Self-Assembly of Fibers and Fibrils. Angewandte Chemie International Edition 2006, 45 (44), 7324-7328, DOI:http://dx.doi.org/10.1002/anie.200602001.
- Danke, V., et al., Structure formation in nanophase-separated systems with lamellar morphology: Comb-like vs. linear precision polymers. European Polymer Journal 2018, 103, 116-123, DOI:https://doi.org/10.1016/j.eurpolymj.2018.03.041. Reimann, S., et al., Synthesis of supramolecular precision polymers: Crystallization under conformational constraints. Journal of Polymer Science Part A: Polymer Chemistry 2017, 55 (22), 3736-3748, DOI:http://dx.doi.org/10.1002/pola.28759.
- Reimann, S., et al., Synthesis and Crystallization of Precision Polymers with Repetitive Folding Elements. Macromolecular Chemistry and Physics 2014, 215 (20), 1963-1972, DOI:http://dx.doi.org/10.1002/macp.201400183.
- Canalp, M. B., et al., Hybrid polymers bearing oligo-l-lysine(carboxybenzyl)s: synthesis and investigations of secondary structure. RSC Advances 2020, 10 (3), 1287-1295, DOI:http://dx.doi.org/10.1039/C9RA09189K.
- Freudenberg, J., et al., Multisegmented Hybrid Polymer Based on Oligo-Amino Acids: Synthesis and Secondary Structure in Solution and in the Solid State. Macromolecules 2019, 52 (12), 4534-4544, DOI:https://doi.org/10.1021/acs.macromol.9b00684.
- Canalp, M. B., et al., Secondary structure of end group functionalized oligomeric-l-lysines: investigations of solvent and structure dependent helicity. RSC Advances 2019, 9 (38), 21707-21714, DOI:http://dx.doi.org/10.1039/C9RA03099A.
- Freudenberg, J., et al., Precision polymers containing main-chain-amino acids: ADMET polymerization and crystallization. RSC Advances 2017, 7 (75), 47507-47519, DOI:http://dx.doi.org/10.1039/C7RA10485E.
- Appiah, C., et al., Crystallization behavior of precision polymers containing azobenzene defects. European Polymer Journal 2017, 97 (Supplement C), 299-307, DOI:https://doi.org/10.1016/j.eurpolymj.2017.10.023. Appiah, C., et al., Synthesis of photoresponsive main-chain oligomers with azobenzene moieties via ADMET oligomerization and their micellization properties. Polymer Chemistry 2017, 8 (18), 2752-2763, DOI:http://dx.doi.org/10.1039/C7PY00426E. Appiah, C., et al., Synthesis and characterization of new photoswitchable azobenzene-containing poly(?-caprolactones). RSC Advances 2016, 6 (8), 6358-6367, DOI:http://dx.doi.org/10.1039/C5RA25216D.
- Evgrafova, Z., et al., Modulation of amyloid β peptide aggregation by hydrophilic polymers. Physical Chemistry Chemical Physics 2019, 21 (37), 20999-21006, DOI:http://dx.doi.org/10.1039/C9CP02683E. Funtan, S., et al., Amyloid beta aggregation in the presence of temperature-sensitive polymers. Polymers MDPI 2016, 8 (5), 178, DOI:https://doi.org/10.3390/polym8050178. Paschold, A., et al., Modulating the Fibrillization of Parathyroid-Hormone (PTH) Peptides: Azo-Switches as Reversible and Catalytic Entities. Biomedicines 2022, 10 (7), 1512 ,DOI:https://doi.org/10.3390/biomedicines10071512 . Sen, N., et al., Membrane Anchored Polymers Modulate Amyloid Fibrillation. Macromolecular Rapid Communications 2021, 42 (12), 2100120, DOI:https://doi.org/10.1002/marc.202100120. Kumar, S., et al., Bifunctional Peptide–Polymer Conjugate-Based Fibers via a One-Pot Tandem Disulfide Reduction Coupled to a Thio-Bromo “Click” Reaction. ACS Omega 2020, 5 (30), 19020-19028, DOI:https://doi.org/10.1021/acsomega.0c02326. Kumar, S., et al., Thio-Bromo “Click” Reaction Derived Polymer–Peptide Conjugates for Their Self-Assembled Fibrillar Nanostructures. Macromolecular Bioscience 2020, 20 (6), 2000048, DOI:https://doi.org/10.1002/mabi.202000048. Kumar, S., et al., Peptide-induced RAFT polymerization via an amyloid-β17–20-based chain transfer agent. Soft Matter 2020, 16 (30), 6964- 6968, DOI:https://doi.org/10.1039/D0SM01169J. Deike, S., et al., β-Turn mimetic synthetic peptides as amyloid-β aggregation inhibitors. Bioorganic Chemistry 2020, 101, 104012, DOI:https://doi.org/10.1016/j.bioorg.2020.104012.
- Evgrafova, Z., et al., Probing Polymer Chain Conformation and Fibril Formation of Peptide Conjugates. ChemPhysChem 2019, 20 (2), 236-240, DOI:https://doi.org/10.1002/cphc.201800867.
- Deike, S., et al., Constraining Polymers into β-Turns: Miscibility and Phase Segregation Effects in Lipid Monolayers. Polymers 2017, 9 (8), 369, DOI:http://www.mdpi.com/2073-4360/9/8/369.
- Deike, S., et al., Induction of Chirality in β-Turn Mimetic Polymer Conjugates via Postpolymerization “Click” Coupling. Macromolecules 2017, 50 (7), 2637-2644, DOI:http://dx.doi.org/10.1021/acs.macromol.7b00343.
- Freudenberg, J., et al., Chirality Control of Screw-Sense in Aib-Polymers: Synthesis and Helicity of Amino Acid Functionalized Polymers. ACS Macro Letters 2020, 686-692, DOI:https://doi.org/10.1021/acsmacrolett.0c00218.
- Rohmer, M., et al., Chiral amines as initiators for ROP and their chiral induction on poly(2-aminoisobutyric acid) chains. Polymer Chemistry 2021, 12 (43), 6252- 6262, DOI:https://doi.org/10.1039/D1PY01021B.