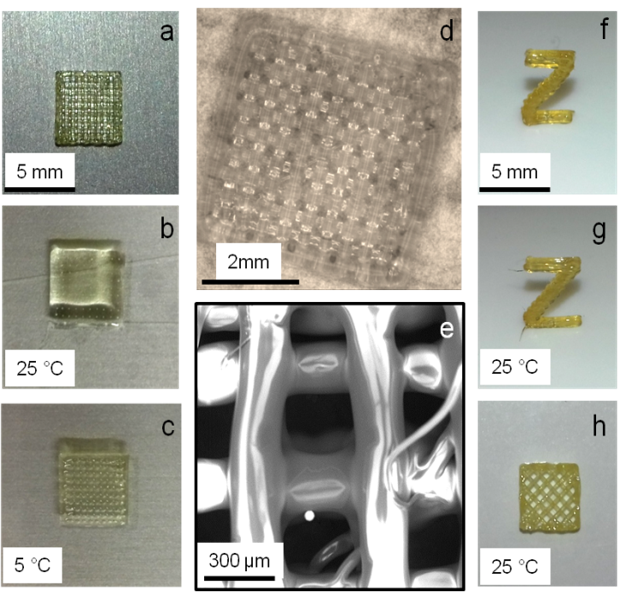
The development on novel materials is directed to on novel battery-electrodes, drug-release systems, stress-sensing-1 and (bio)-degradable soft/hard interfaces. Three dimensional (3D)-printing technology has become societies leading method to form materials for many applications. Prominent examples are the 3D- printing of self-healing polymer systems, pharmaceutical delivery materials and capsule-based multicomponent materials. Printing biodegradable polymers, capsules and materials for triggered time-release with defined long term delivery profiles represents an important challenge, currently addressed by proper design and synthesis of 3D-printable (co)-polyesters. We use multimode 3D-printing methods to prepare modern materials, with embedded self-healing-, mechanochromic- and pharmaceutical function2. 3D-printing enables to print drug-delivery systems, electrolytes and multifunctional materials with stress-diagnostic and self-healing properties.3-9
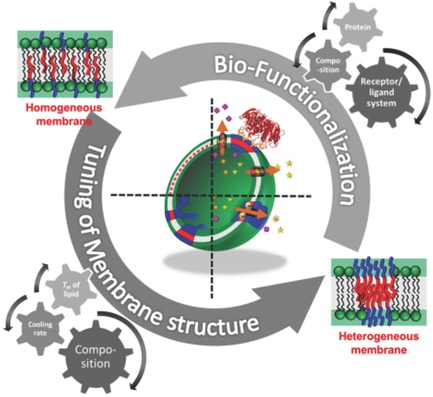
Delivery of pharmaceutical cargo as nanosized particles, vesicles or aggregates has become a prominent and medical useful endeavor.10, 11 Polymers and lipids, equipped with modern STEALTH-technology are particularly interesting in this context, termed vesicles or polymersomes.12-15 Especially in view of site-specific delivery and imaging technology small, STEALTH-like particles with embedded functionalities are important, mediating both, biocompatibility and delivery of drugs, dyes and recognition sites. Based on our longstanding experience on artificial and biological membranes,10, 11, 14, 16 we are currently developing ultra-small nanoparticles for high resolution imaging technology. Polymeric carrier-molecules consisting of a single chain are used in photoacoustic spectroscopy, displaying prolonged circulation times and excellent imaging properties.110
References
- Döhler, D., et al., Qualitative sensing of mechanical damage by a fluorogenic “click” reaction. Chemical Communications 2016, 52 (74), 11076-11079, DOI:http://dx.doi.org/10.1039/C6CC05390D.
- Rupp, H., et al., 3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromolecular Rapid Communications 2019, 1900467, DOI:https://doi.org/10.1002/marc.201900467.
- Hilgeroth, P.S., et al., 3D-printing of Triamcinolone Acetonide in Triblock Copolymers of Styrene-Isobutylene-Styrene as a Slow Release System, Polymers, 2022, 14, 3742, DOI:https://doi.org/10.3390/polym14183742. Du, F., et al., 3D-printing of the polymer/insect-repellent system poly(l-lactic acid)/ethyl butylacetylaminopropionate (PLLA/IR3535). International Journal of Pharmaceutics 2022, 624, 122023, DOI:https://doi.org/10.1016/j.ijpharm.2022.122023. Rupp, H., et al.,3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromolecular Rapid Communications 2019, 40 (24), 1900467, DOI:https://doi.org/10.1002/marc.201900467.
- Rupp, H., et al., 3D Printing of Solvent-Free Supramolecular Polymers. Frontiers in Chemistry 2021, 9, 771974, DOI:https://doi.org/10.3389/fchem.2021.771974.
- Binder, W. H., et al., Kunststofftechnik: Thermischen Abbau erkennen. Nachrichten aus der Chemie 2022, 70 (1), 40-41, DOI:https://doi.org/10.1002/nadc.20224120686.
- Katcharava, Z., et al., 3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability. Nanomaterials 2022, 12 (11), 1859, DOI:https://doi.org/10.3390/nano12111859.
- Rupp, H., et al., Printable Electrolytes: tuning 3D printing by multiple hydrogen bonds and added inorganic lithium-salts (LiTFSI). Advanced Materials Technology 2022, 7(8), 2200088, DOI:https://doi.org/10.1002/admt.202200088.
- Rupp, H., et al., Multicomponent Stress-Sensing Composites Fabricated by 3D-Printing Methodologies. Macromolecular Rapid Communications 2021, 2000450, DOI:https://doi.org/10.1002/marc.202000450. Rupp, H., et al., 3D Printing of Core–Shell Capsule Composites for Post-Reactive and Damage Sensing Applications. Advanced Materials Technologies 2020, 2000509, DOI:https://doi.org/10.1002/admt.202000509.
- Döhler, D.; Kang, J.; Cooper, C. B.; Tok, J. B. H.; Rupp, H.; Binder, W. H.; Bao, Z. Tuning the Self-Healing Response of Poly(dimethylsiloxane)-Based Elastomers. ACS Applied Polymer Materials 2020, 2 (9), 4127-4139, DOI:https://dx.doi.org/10.1021/acsapm.0c00755.
- Schulz, M., et al., Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromolecular Rapid Communications 2015, 36 (23), 2031-2041, DOI:http://dx.doi.org/10.1002/marc.201500344.
- Binder, W. H., et al., Domains and Rafts in Lipid Membranes. Angewandte Chemie International Edition 2003, 42 (47), 5802-5827, DOI:http://dx.doi.org/10.1002/anie.200300586.
- Fuchs, C., et al., Molecular arrangement of symmetric and non-symmetric triblock copolymers of poly(ethylene oxide) and poly(isobutylene) at the air/water interface. Journal of Colloid and Interface Science 2015, 437 (0), 80-89, DOI:http://dx.doi.org/10.1016/j.jcis.2014.09.050.
- Schulz, M., et al., Beyond the Lipid-Bilayer: Interaction of Polymers and Nanoparticles with Membranes. Soft Matter 2012, 8 (18), 4849–4864, DOI:http://dx.doi.org/10.1039/C2SM06999G. Olubummo, A., et al., Phase Changes in Mixed Lipid/Polymer Membranes by Multivalent Nanoparticle Recognition. Langmuir 2014, 30 (1), 259-267, DOI:http://dx.doi.org/10.1021/la403763v. Schulz, M., et al., Hybrid lipid/polymer giant unilamellar vesicles: effects of incorporated biocompatible PIB-PEO block copolymers on vesicle properties. Soft Matter 2011, 7 (18), 8100-8110, DOI:http://dx.doi.org/10.1039/C1SM05725A.
- Olubummo, A., et al., Controlling the Localization of Polymer-Functionalized Nanoparticles in Mixed Lipid/Polymer Membranes. ACS Nano 2012, 6 (10), 8713–8727, DOI:http://dx.doi.org/10.1021/nn3023602.
- Schulz, M., et al., Lateral surface engineering of hybrid lipid-BCP vesicles and selective nanoparticle embedding. Soft Matter 2014, 10 (6), 831-839, DOI:http://dx.doi.org/10.1039/C3SM52040D. Sachsenhofer, R., et al., Polymersome-Embedded Nanoparticles. Macromol. Symp. 2007, 254 (1), 375-377, DOI:https://doi.org/10.1002/masy.200750854. Binder, W. H., et al., Guiding the location of nanoparticles into vesicular structures: a morphological study. Phys. Chem. Chem. Phys. 2007, 9 (48), 6435-6441, DOI:https://doi.org/10.1039/B711470M. Binder, W. H. Supramolecular Assembly of Nanoparticles at Liquid-Liquid Interfaces. Angew. Chem., Int. Ed. 2005, 44 (33), 5172-5175, DOI:https://doi.org/10.1002/anie.200501220.
- Schulz, M., et al., Controlling Molecular Recognition with Lipid/Polymer Domains in Vesicle Membranes. Angewandte Chemie International Edition 2013, 52 (6), 1829-1833, DOI:http://dx.doi.org/10.1002/anie.201204959.
- Thümmler, J. F., et al., Tuning the Internal Compartmentation of Single-Chain Nanoparticles as Fluorescent Contrast Agents. Macromolecular Rapid Communications 2022, 2200618, DOI:https://doi.org/10.1002/marc.202200618. Roos, A. H., et al., Nanoscale structure and dynamics of thermoresponsive single-chain nanoparticles investigated by EPR spectroscopy. Soft Matter 2021, 17 (29), 7032-7037, DOI:https://doi.org/10.1039/D1SM00582K. Hoffmann, J. F., et al., Fluorescent and Water Dispersible Single-Chain Nanoparticles: Core–Shell Structured Compartmentation. Angewandte Chemie International edition 2021, 60 (14), 7820-7827, DOI: https://doi.org/10.1002/anie.202015179.