β-Turn mimetic synthetic peptides as amyloid-β aggregation inhibitors.
Deike, S., et al. Bioorganic Chemistry 2020, 101, 104012, https://doi.org/10.1016/j.bioorg.2020.104012
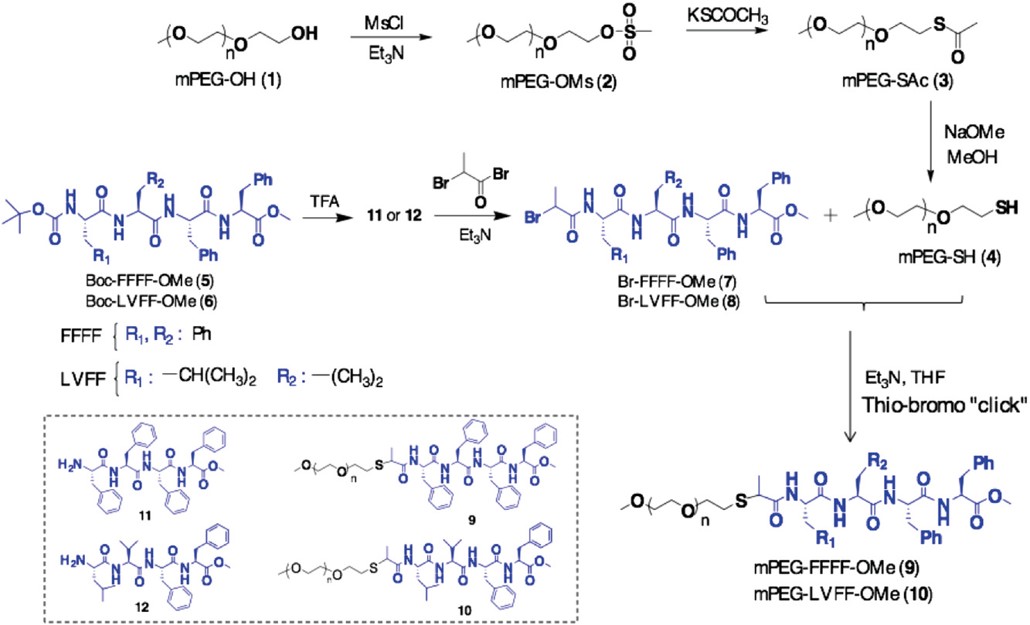
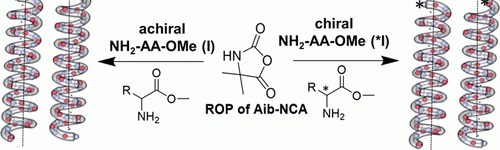
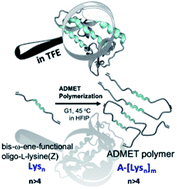
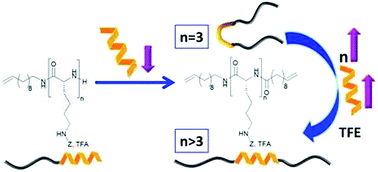
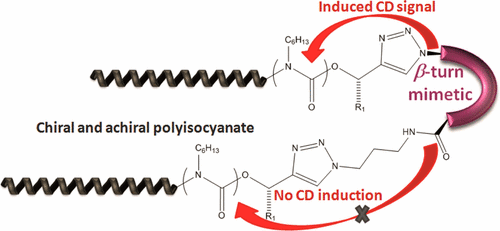
Aggregation of amyloid peptides results in severe neurodegenerative diseases. We here report the preparation of beta-turn mimetic conjugates containing synthetic turn mimetic structures in the turn region of Aβ40 and Aβ16-35, replacing 2 amino acids in the turn-region G25 – K28. The structure of the turn mimic induces both, acceleration of fibrillation and the complete inhibition of fibrillation, confirming the importance of the turn region on the aggregation. Reproduced with permission. Copyright 2020© Elsevier B. V.