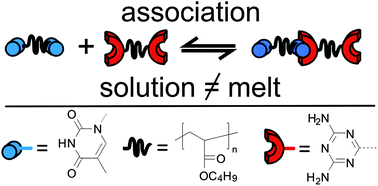
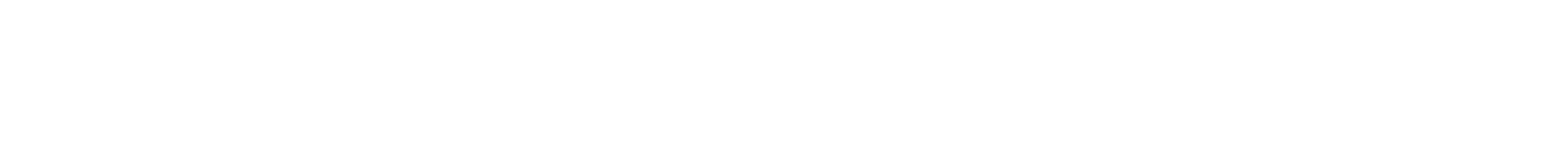Synthesis of an Amphiphilic β-Turn Mimetic Polymer Conjugate.
Malke, M., et al. ACS Macro Letters 2014, 4 (3), 393-397, DOI: http://pubs.acs.org/doi/abs/10.1021/mz500108n.
A new biomimetic polymer containing a beta-turn mimetic element (1) was synthesized, using a combination of living carbocationic polymerization (LCCP), amidation, and “click” chemistry. By means of size exclusion chromatography (SEC), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), NMR spectroscopy, and LC/MALDI-TOF MS, a detailed structural proof of the β-turn mimetic PIB conjugate (1) was possible. Reproduced with permission. Copyright 2014©, American Chemical Society.